Cell culture products
We meet emerging market needs by developing products based on our core cell culture medium and culture bag technologies.
FUKOKU has been manufacturing cell culture bag products since 2003. Products with superior safety and quality are required for use as the cell culture containers used during the cell culture process in cell therapy.
We continued to develop unique products in desire to bring closed systems to the cell culture process. In addition to cell culture bags, we also provide products such as cell culture media, cell culture systems.
All of these products are produced in class 1000 or class 10000 cleanroom environments.
Cell culture bags
Developed for immune cell therapy research, the bags are also ideal for use as culture containers for lymphocytes and other floating cells. They are made from highly gas-permeable film for high-density cultures. The highly transparent film also allows for microscopic observation.
Closed system bags can be used to reduce the risk of contamination or infection by foreign substances, bacteria, and viruses.
They are made from material compliant with the Japanese Pharmacopoeia plastic medical and pharmaceutical product container testing methodology, as well as sterilized transfusion set standards.
We can also customize the shapes, tubes, and ports of single-use bags (gas barrier properties).
We also develop cell cryopreservation bags that offer superior anti-shock performance in extremely low temperatures (-196°C). Please contact us for details.
Cell culture bag product lineup
| Product | FKCB-ST3 | FKCB215 | FKCB-215C anti-CD3 antibody (coat bag) |
|
|---|---|---|---|---|
| Shape |  |
 |
 |
|
| Culture area | Length Width Volume |
292mm 220mm 〜1200ml |
183mm 120mm 〜350ml |
183mm 120mm 〜350ml |
| Tubes | Filling tube Sampling tube Connection tube |
Filling tube Sampling tube |
Filling tube Sampling tube |
|
| Suggested retail price/bag | Please contact us | |||
| Quantity per box | 10 | 10 | 10 | |
*Larger cultures can be grown by connecting FKCB 215 and FKCB-ST3.
Contact us about cell culture bags
Contact us
Please submit any inquiries here.
Cell culture media
FUKOKU develops and manufactures a range of cell culture media, and provides products with superior proliferative response suited to the type of cell and application.
We can provide filled cell culture bags for use in suspension cell culture applications. A closed cell culture can help reduce the risk of contamination or infection by foreign substances, bacteria, or viruses. We can also substitute the material for one with gas barrier properties for single-use bag applications, change the tube diameter/length, or customize port specifications (for example, changing the product from a needle-type product to a needleless product).
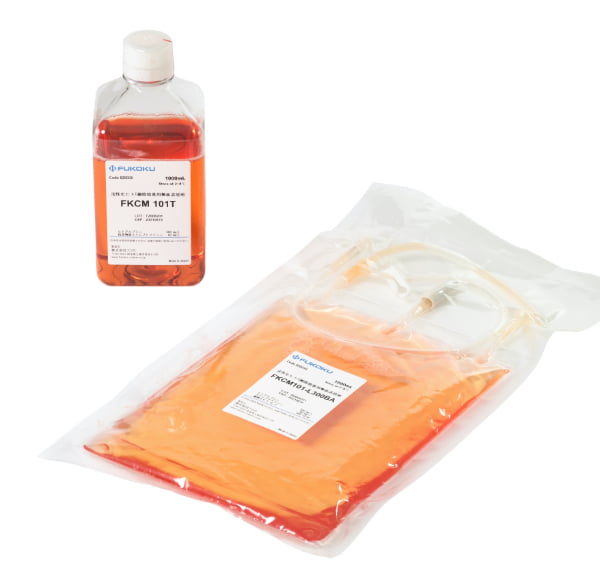
FKCM 101
Liquid culture medium for human T cell activation and expansion
Use: Used for human peripheral blood lymphocyte activation cultures.
- Includes no proteins other than human serum albumin
- Includes streptomycin as an antibiotic
- Adding 4% serum during primary culturing can promote cell proliferation
- Culture can grow without serum during expansion culturing
- Bags are made from materials with superior gas permeability, and their safety and quality complies with the method for plastic containers of the JAPAN pharmacopoeia.
- Regenerative medicine, etc. product material eligibility confirmed (Medical Device Inspection Office Notice No. 1122004)
- US DMF registration (No.MF040316 : Cell Culture Medium)
| Product lineup | IL-2 content | Volume |
|---|---|---|
| FKCM 101T | No IL-2 | 1000ml |
| FKCM 101B | No IL-2 | 1000ml |
| FKCM101-L300T | IL-2 300 IU/ml | 1000ml |
| FKCM101-L300BA | IL-2 300 IU/ml | 1000ml |
| FKCM101-L13T | IL-2 1300 IU/ml | 1000ml |
*MF registered (No. 230MF40005)
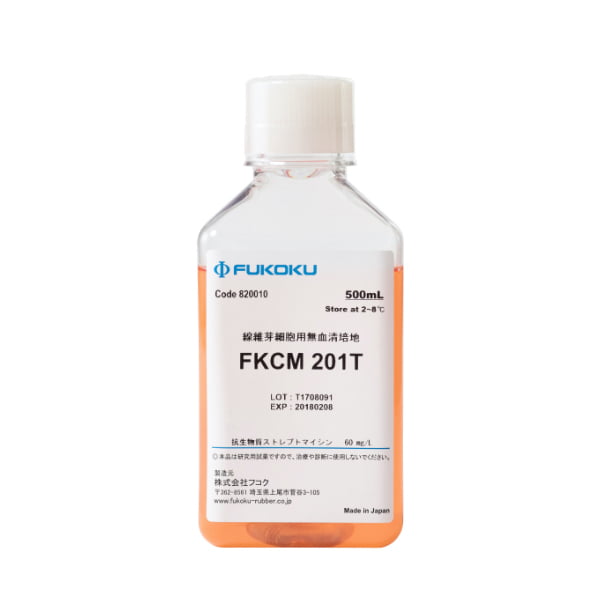
FKCM 201
Serum-free medium for human fibroblasts
Use: Contains components required for human fibroblasts to proliferate and maintain functionality, for use in researching wound healing, skin conditions, etc.
- Medium materials contain no animal by-products
- Includes streptomycin as an antibiotic
- No need to add cell-adhesion factors such as collagen during primary culturing
- Adding 0.5% to 2% serum can promote cell proliferation
- US DMF registration (No.MF040316 : Cell Culture Medium)
| Product lineup | Volume | Packaging |
|---|---|---|
| FKCM 201T | 500ml | Plastic bottle |
FKCM 301
Serum-free medium for human mesenchymal stem cells
Use: Contains components required for mesenchymal stem cells to proliferate and maintain functionality, for use in researching regenerative medicine, immunological conditions, etc.
- Medium materials contain no animal by-products
- Includes streptomycin as an antibiotic
- No need to add cell-adhesion factors such as collagen during primary culturing
- Adding 0.5% to 2% serum can promote cell proliferation
- Regenerative medicine, etc. product material eligibility confirmed (Medical Device Inspection Office Notice No. 1122004)
- US DMF registration (No.MF040316 : Cell Culture Medium)
| Product lineup | Volume | Packaging |
|---|---|---|
| FKCM 301T | 500ml | Plastic bottle |

Contact us about cell culture media
Contact us
Please submit any inquiries here.